The Mitophagy Mystery: Discovering the Solution for Cellular Aging
Groundbreaking research reveals how a cellular recycling process may hold the key to combating age-related diseases
mitochondria = powerhouse of the cell; mitophagy = selective recycling of mitochondria
A groundbreaking discovery has shed new light on the mechanisms of aging.1 Researchers have uncovered a crucial link between a cellular recycling process called mitophagy and the onset of cellular senescence, a hallmark of aging. This finding not only deepens our understanding of how cells age but also opens up exciting possibilities for developing therapies to combat age-related diseases.
At the heart of this research is mitophagy, a quality control mechanism that cells use to remove damaged or dysfunctional mitochondria. Mitochondria, often referred to as the powerhouses of the cell, are essential for energy production and cellular health. When these organelles become damaged or inefficient, they can contribute to cellular dysfunction and aging. Mitophagy acts as a cellular housekeeping service, ensuring that only healthy mitochondria remain operational.
The study, conducted by a team of international researchers, reveals that primary human cells maintain remarkably high levels of basal mitophagy. This was a surprising discovery, as previous research using immortalized or cancer cell lines had shown low or undetectable levels of mitophagy in unstressed conditions. The researchers found that this high basal mitophagy is critical for maintaining cellular health and preventing the onset of senescence.
One of the most striking findings of the study is that mitophagy is dramatically suppressed during cellular senescence and aging. As cells enter a senescent state, either through stress-induced damage or natural aging processes, their ability to perform mitophagy diminishes significantly. This suppression occurs early in the senescence process, suggesting that it may be a driving force behind cellular aging rather than just a consequence.
The research team identified the molecular players involved in this process, uncovering that basal mitophagy in primary human cells is controlled by the PINK1/Parkin/p62-dependent pathway. This pathway, previously thought to be activated only in response to severe mitochondrial damage, was found to contribute significantly to the basal turnover of mitochondria in healthy cells. The loss of this pathway's function was sufficient to trigger cellular senescence, highlighting its critical role in maintaining cellular youth.
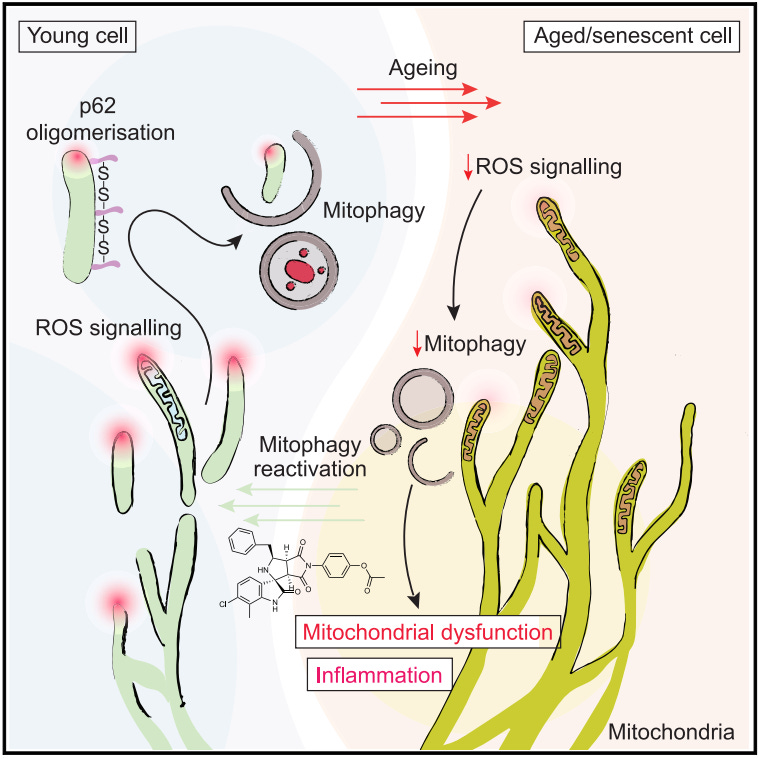
Intriguingly, the study revealed that mitochondrial reactive oxygen species (ROS) act as a trigger for mitophagy. In healthy cells, mitochondria producing high levels of superoxide were found to be selectively targeted for degradation. However, during senescence, changes in mitochondrial dynamics and respiration led to a loss of this ROS signaling, contributing to the suppression of mitophagy.
The consequences of mitophagy suppression are far-reaching. As cells lose their ability to remove damaged mitochondria, they accumulate dysfunctional organelles, leading to a cascade of cellular problems. This accumulation contributes to changes in cell morphology, metabolism, and function that are characteristic of senescent cells. The researchers observed increased cell size, alterations in nuclear structure, and the development of a senescence-associated secretory phenotype (SASP) in cells with impaired mitophagy.
Perhaps the most exciting aspect of this research is its potential therapeutic implications. The study demonstrated that pharmacological interventions capable of restoring mitophagy could reverse many of the cellular aging phenotypes. NAD precursors, such as nicotinamide (NAM) and nicotinamide riboside (NR), as well as the autophagy activator rapamycin, were shown to rescue mitophagy defects and reduce markers of senescence.
Furthermore, the research team identified a novel small molecule, STOCK1N-57534, that targets the p62 protein involved in the mitophagy process. This compound was able to restore mitophagy in senescent cells and cells from aged donors, leading to a reduction in various aging markers. The success of this small molecule opens up new avenues for developing targeted therapies to combat cellular aging.
The implications of this research extend far beyond the laboratory. As our global population continues to age, understanding the mechanisms of cellular aging becomes increasingly crucial. Age-related diseases, such as neurodegenerative disorders, cardiovascular diseases, and cancer, pose significant challenges to healthcare systems worldwide. By uncovering the role of mitophagy in cellular aging, this study provides a new target for developing interventions that could potentially delay or prevent the onset of these age-related conditions.
However, it's important to note that while these findings are exciting, they also raise new questions. The research primarily focused on cell culture models, and translating these results to whole organisms, especially humans, will require further investigation. Additionally, the complex interplay between mitophagy and other cellular processes involved in aging needs to be explored in greater detail.
The study also highlights the importance of using relevant cellular models in aging research. The discovery that primary human cells exhibit high levels of basal mitophagy, in contrast to commonly used immortalized cell lines, underscores the need for human-centric approaches in studying cellular aging. This finding challenges some of the existing paradigms in the field and emphasizes the importance of validating results across different cellular models.
As we look to the future, this research opens up several promising avenues for further exploration. Understanding how mitophagy interacts with other cellular stress response pathways, investigating its role in different tissues and organs, and exploring how environmental factors influence this process are all critical areas for future study. Moreover, developing more sophisticated methods to measure and modulate mitophagy in living organisms could pave the way for translating these findings into clinical applications.
The potential impact of this research on human health and longevity cannot be overstated. If therapies based on enhancing mitophagy prove successful, we could see a future where age-related diseases are managed more effectively, leading to improved quality of life in our later years. It's conceivable that such interventions could not only treat existing conditions but also prevent or delay the onset of age-related decline.
This groundbreaking study has unveiled mitophagy as a critical player in the complex drama of cellular aging. By demonstrating how this cellular recycling process influences senescence and identifying potential ways to modulate it, the researchers have opened up exciting new possibilities in the field of aging research. As we continue to unravel the mysteries of cellular aging, discoveries like these bring us one step closer to understanding and potentially controlling the aging process.
TLDR;
So here’s what we’ve learned in this article:
The study discovered that primary human cells maintain high levels of basal mitophagy, unlike immortalized or cancer cell lines.
During cellular senescense, mitophagy is significantly downregulated in cells from aged donors.
The pathway known as the PINK1/Parkin/p62-dependent pathway controls basal mitophagy in primary human cells. This was originally thought to be activated only in severe mitochondrial damage.
Mitochondrial-derived reactive oxygen species act as a signal for basal mitophagy initiation.
Mitophagy suppression occurs early in the senescense process, suggesting it’s a driver rather than a consequence of cellular aging.
Loss of mitophagy leads to accumulatio of dysfunctional mitochondria and triffers cellular senscence.
NAD precursors (NAM, NR) and rapamycin can rescue mitophagy defects and reduce senescence markers.
STOCK1N-57534 was identified as a compound that can restore mitophagy in senescent and aged cells, reversing several aging phenotypes.
Targeting mitophagy could be a promising approach for developing anti-aging interventions.
Kelly et al., Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule, Developmental Cell (2024), https://doi.org/10.1016/j.devcel.2024.04.020




This is an intersting find and also something David Sinclair picked up on. I tried to find what foods contain STOCK1N-57534, but I was not able to find anything. Do you have any idea where we can find this naturally?